Ionic solvation effects
 EDLs
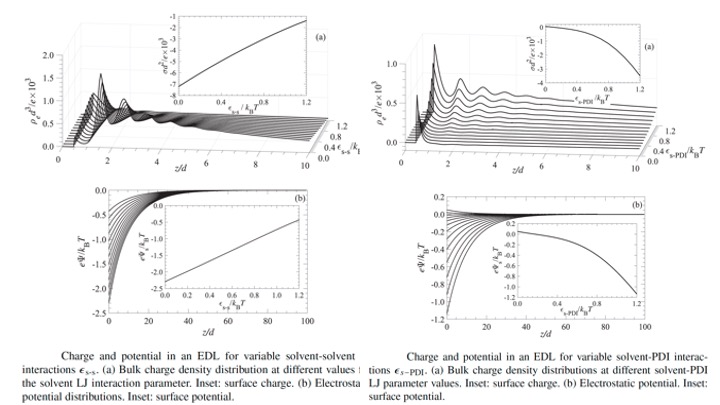
EDLsElectric double layers are complex systems that involve a wide variety of interactions between the different components of the electrolyte solutions and with the charged interface. While the role of all Coulombic types of interactions is clear, that of the non-Coulombic forces is less obvious. The focus in the present study is on the effect of bulk solvation interactions on the properties of the electric double layer. The analysis is based on classical density functional theory. This approach allows us to account for the correlations between all charged (ionic) and uncharged (solvent) species in the solution. The surface charge at the boundary of the electric double layer is derived from the surface chemistry pertinent to the system. The surface is sensitive to the concentration of potential determining ions, which in turn depends on the correlations and activities of all remaining components. The analysis shows that the solvation forces have a profound effect on the charge and potential distributions in an electric double layer. This is true not just for the solvation of the potential determining ions, but for all species. Even varying the solvent-solvent interaction has a significant impact on the charge and potential distributions in the electric double layer.